- 1. Patient Preparation
- 2. Proper Identification of Specimens
- 3. Test Requisition
- 4. Specimen Separation And Storage Guidelines
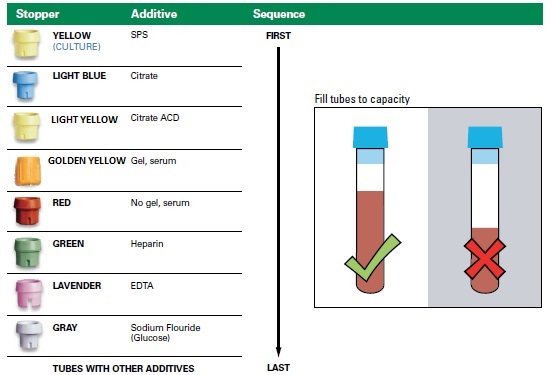
- 5. Specimen Collection Tubes
- 6. Order of draw
- 7. Specimen Packaging
Patient Preparation
Many tests require specific patient preparation (i.e., fasting, diets, urine voiding). If you have questions about patient preparation for any test, please consult this Directory or call Client Services for further assistance.
Fasting Requirements
A fasting specimen is preferred for the majority of tests performed on serum, plasma or whole blood. Non-fasting specimens often contain fat particles that can interfere with many analytical procedures. Refer Common Causes of Unacceptable Blood Specimens and Inaccurate Test Results (Turbidity) in the Blood, Urine and Stool section. Fasting is defined as no consumption of food or beverage, other than water, for at least 10 to 12 hours before testing.
Provocation Tests
Some tests require the patient to ingest a substance. The most common are the Glucose Tolerance Tests where the patient drinks a solution containing glucose, and blood samples are obtained before and at various times after the drink, to measure the concentration of glucose in plasma or serum. In the standard Glucose Tolerance Tests, adults ingest 75 g (10 ounces) of a glucose solution. Children ingest an amount of glucose proportional to their body weight. (1.75 grams of glucose per kilogram of body weight, up to 75 g of glucose)
Specimen Labels
Each specimen submitted must have a label. This label must include at a minimum of two identical items: the patient’s name, written exactly as it appears on the test requisition (e.g., Doe, John), TRF Number (provided by the Lab in the Test Requisition Form), Patient Date of Birth, etc. Be sure that the label is securely attached. If the label is hand-written, use a ballpoint pen. Do not use a felt tip pen. When submitting a specimen in a container other than the tube used to draw the sample (e.g., transfer vials), also indicate the specimen type on the label (e.g., serum, plasma, urine, etc.). When submitting specimens for microbiological testing (e.g., cultures, bacterial antigen, microscopic examination) the nature of the sample and the specific organism(s) to be detected, if any, should be specified.
Test Requisition
Specimens must be accompanied by a paper requisition, prepared either by hand or printed from an electronic ordering system. The requisition, at a minimum should contain the following information:
• Adequate patient identification information (e.g., name, address, telephone number, medical record number)
• Patient gender
• Patient date of birth, or age
• Name and address of the physician ordering the test
• Test(s) requested
• Date of specimen collection, when appropriate
• Source and type of specimen and time, when appropriate
• Clinical information, when appropriate
Complete the “Patient Information” and “Insurance Information” sections on the requisition. Select the tests to be performed. In the case of specimens submitted for microbiological testing (e.g., cultures, bacterial antigen, microscopic examination) the nature of the sample and the specific organism(s) to be detected, if any, should be specified. When ordering tests in a series (e.g., growth-hormone stimulation, glucose tolerance, etc.):
1. Use one test requisition.
2. Label each specimen with the patient’s name, date and time, or site (if applicable).
3. Write the number of specimens on the test requisition.
4. Submit all specimens within a series together in one specimen bag.
Improperly labelled specimens will be rejected.
It is important to note that when Plasma/Serum are the sample required for the test, whole blood samples should not be sent.
Whole blood: The most common test using anticoagulated whole blood is the Complete Blood Count (CBC) and blood film morphology, which should be collected using a lavender-top (EDTA) plastic vacuum tube. Other tests might require anticoagulants such as green-top (heparin) or light blue-top (sodium citrate) tube. Please follow instructions for the individual test. Collect an adequate volume of blood. Fill the tube to capacity (fill mark on the tube), since the partial filling will result in distortions caused by the osmolality of the anticoagulant. Under filled or overfilled blood collection tubes will not be accepted for testing. Immediately mix the blood thoroughly with the additives by gently inverting eight (8) times, or four (4) times when using light blue-top (sodium citrate) tubes. Incomplete mixing or delay in mixing after phlebotomy will result in microscopic partial clotting of the sample, which can cause spuriously low platelet counts. Maintain the specimen at room temperature or on cold packs before submitting to our laboratory, unless instructed otherwise by the specimen requirement information in this Directory or by the laboratory. Never freeze whole blood unless it is specifically instructed in the specimen requirement instructions. If you store cold packs in the freezer, be sure to allow sufficient time for them to warm to refrigerator temperature before placing whole blood specimens near them. To minimize the risk of hemolysis, do not place whole blood specimens in direct contact with cold packs. CBC samples should reach to the laboratory within 24 hours of collection.
Heparin-whole blood: Common use of sodium heparin tube is for chromosomal analysis of blood and/or bone marrow. This is usually for the investigation of hematologic disorders/malignancies. In case of Blood Specimen, 5-10 mL total volume is preferred but volume < 3 mL calls for rejection. In case of bone marrow minimum 2 mL is recommended. Do not use lithium heparin for any serum metal testing.
Plasma: Blood collection is to be carried out using standard Vaccutainer tubes & collection devices selected for suitable anticoagulant for the tests of interest, provided by Thumbay Labs. This avoids under filling or overfilling of tubes and in turn, ensures adequate anticoagulation. Overfilling can result into inadequate anticoagulation and can result into clot or micro a clot formation. Under filling will lead to an excess of anticoagulant and can affect quality of plasma. Mix the collected whole blood specimen in tubes containing anticoagulant by gently inverting for few times as recommended in Table-2.
Centrifuge the sample at RT, 3500 RPM or 1250 RCF for 15 min as early as possible after blood collection. It is recommended that plasma should be separated from cells within 60 min of collection. Platelet poor plasma (plasma platelet < 10,000/µL) is a mandatory requirement for all coagulation tests and would require double centrifugation i.e. recentrifugation of sample/obtained plasma for the same duration and at the same speed. Though samples are collected in Sodium Fluoride & Oxalate containing tube for Plasma Glucose, glucose levels decrease by 7-9 mg during first hour. Though many routine chemistry tests can be performed from heparinized plasma, serum is recommended. Values obtained using such plasma could be higher by 3% as compared to Serum.
Platelet Poor Plasma: Collect blood in a light blue-top (sodium citrate) tube and mix the tube by inverting the tube gently four (4) times. Centrifuge at 2,500–3,500 RPM for 15 minutes. Using a plastic pipette, remove plasma taking care to avoid the buffy coat layer containing leukocytes and platelets. Transfer to a plastic tube. Centrifuge a second time at 2,500–3,500 RPM for 15 minutes. Using a new plastic pipette, remove the plasma taking care to avoid the layer at the bottom of the tube. Transfer plasma to a properly labeled, clean plastic screw-cap vial and attach the label from the lower portion of the test requisition, if applicable. Cap firmly to prevent leakage. Write “PP PLASMA” on the plastic screw-cap vial label and on the test requisition to ensure that Serum or Plasma is separated as described below, within the required time limits.
Serum: When blood is collected in Plain Tube (Red Top) without clot activator, allow it to stand at Room Temperature (RT), keeping it vertical. It takes about 30-60 min for blood to clot completely. When blood collected in plain tube with Clot Activator (Golden cap), it takes about 15-30 min to clot completely. After ensuring that there is complete clotting, please centrifuge the sample at RT, 3500 RPM or 1250 RCF for 10-15 min; immediately after centrifugation remove separated serum using clean transfer pipette into transport vial. Separate serum from cells no later than 60 min after collection. Samples collected in SST tube require to be centrifuged. Following centrifugation it can be transported as it is. It is recommended to preserve primary tube (in which the sample was collected) till reports are released. The vial into which the serum is transferred, also called secondary tube, must be labeled identically as was the primary tube and the information must match that transcribed on Test Request Form.
Blood Collection Tubes The use of vacutainers with appropriate collection devices is bly recommended so that blood volume matches with the amount of additive. Please ensure that selected collection tube is within expiry period stated on the tube.
There are universal color codes for various commercially available blood containers to indicate presence and type of anticoagulant/additive.

Specimen Labelling
Label each specimen/sample with minimum of two identifications (name/initials, TRF ID, Hospital ID, Date of Collection and Date of Birth & Gender of the patient). It is important to insure that the patient information on the label and the test request form (TRF) match with a minimum of two identifications. Samples which have a mismatch between the label and the accompanying TRF are liable to be rejected.

Specimen Packaging
The following are the minimum specimen packaging guidelines that should be followed when submitting specimens.
1. Ensure that all specimen container caps and lids are properly tightened to prevent leakage.
2. Properly complete the Test requisition form.
3. Collect the specimen(s) in a proper transport container.
4. Fold the top copy (original) of the test requisition in half widthwise (top to bottom) with the patient’s name and bar code facing out. Retain the second copy for your files.
5. The specimen bag has two pouches. Place the specimen container(s) in the rear pouch (printed side) and the test requisition in the front pouch.
6. Use single pouch for each patient specimen. Do not mix multiple patient specimen in a single pouch.
Holding and Securing Specimens
While awaiting pick-up by a Thumbay Labs messenger, maintain specimens at room temperature or on cold packs unless otherwise noted under the “Transport Temperature” or other specimen requirement in this section or in the Test Listing section. Thumbay Labs has provided lock boxes to the Thumbay Labs messengers for specimen pick-up. However, customers are responsible for the security of specimens prior to pick up.
Frozen Specimens
Frozen specimens must be transported in insulated containers surrounded by an ample amount of frozen ice packs to keep the specimen frozen until it reaches the laboratory. Thawed specimens are unsuitable for analysis. In the event a thawed specimen is received, you will be asked to resubmit an adequate specimen. If you would like more information about sending specimens to Thumbay Labs, please contact our Client Service Representative.
Transporting Specimens to Thumbay Labs
Needles, Sharp Objects or Medical Waste
Do not send any needles or other sharp or breakable objects. Do not send medical waste as a diagnostic specimen since it may violate the law and create a health hazard. Properly discard used needles or other sharp objects prior to transport. Please note for tests requiring the submission of syringes, the needle must be removed and the syringe capped before sending to the laboratory. Ensure that there is no leakage from or visible contamination outside the specimen container.
Transportation
The specimens picked up from the clients end shall be transported in an appropriate temperature condition in sample transportation bags with biohazard symbol. All specimens shall be treated as infections substances.
Thumbay Labs Bike messengers have been provided with temperature controlled transport boxes for specimen collection from the Clients.
Specimen Tracking
Thumbay Labs follow strict compliance on specimen chain of custody from the client end to specimen reaching to laboratory. When the specimens are picked up from the client end the specimen details and time of pick up shall be noted in the Chain of Custody form. Upon receipt of samples in the laboratory the sample transportation time received time and condition shall be reviewed along with number of sample picked up from client end and delivered to laboratory. Thumbay Labs comply to zero lost sample policy during transportation.


